Quick links
- Exploring the apical specialization and calcium signaling pathways in Apicomplexans
- Development of an in vitro culture system for Cryptosporidium parvum
- Strain specific differences in intracellular survival of Toxoplasma gondii
- New therapeutics for toxoplasmosis
- Cellular immunity and control of Toxoplasma
- Discovery of novel Toxoplasma gondii secreted effectors
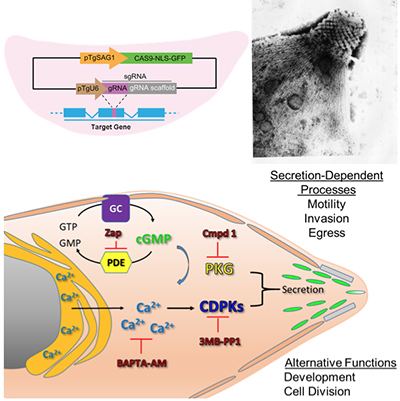
Exploring the apical specialization and calcium signaling pathways in Apicomplexans
Toxoplasma gondii is a model system for studying the unique biology of apicomplexans, including signaling pathway as that control protein secretion, and the apical specializations that define the phylum. CRISPR/Cas9 offers unprecedented efficiency for genome editing that can be used for tagging, modifying, or deleting genes in T. gondii (PMID 24825012). We are exploiting this system to study key aspects of the biology of apicomplexans including:
- The role of a unique family of plant-like calcium dependent protein kinases CDPKs in controlling invasion and egress (PMID:19527888)
- The role of cyclic nucleotide turnover, and regulated kinases such as PKG, in controlling protein secretion (PMID: 28465425)
- The role of apical proteins involved in calcium binding and signaling (PMID: 28475612)
- The release of calcium from intracellular storage sites and downstream signaling pathways that regulate motility
- The composition of the conoid, a unique microtubule organizing center that defines the apical end
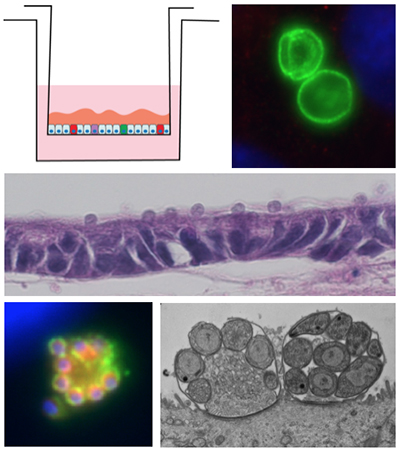
Development of an in vitro culture system for Cryptosporidium parvum
- We are investigating cell culture methods for the continuous in vitro propagation of Cryptosporidium parvum, a protozoan parasite that causes severe gastrointestinal disease in the developing world (PMID: 23680352). Currently, in vitro models only support short-term parasite growth, which necessitates the propagation of Cryptosporidium through animals. These obstacles have hindered the development of genetic tools for the parasite, as well investigation sof host pathogen interactions
- We are exploring the use of primary intestinal epithelial cell lines as an improved system for exploring the Cryptosporidium life cycle. Suing a newly defined newly defined culture system, we have devised methods for continuous propagation of the parasite in vitro. We are utilizing the in vitro cultivation system to develop genetic tools, cell biological investigations of host-pathogen interactions, and evaluation of compounds that block growth and development
- Our work on in vitro cultivation systems for Cryptosporidium is supported by the Bill and Melinda Gates Foundation (https://www.gatesfoundation.org/)
Strain specific differences in intracellular survival of Toxoplasma gondii
- Toxoplasma strains possess strain specific virulence determinants that allow them to override innate host immune mechanisms. We have previously identified such determinants using forward genetic crosses, quantitative trait locus (QTL) mapping, and reverse genetic approaches (PMID 27359216). These studies have defined a family of secretory kinases discharged form the rhoptries (ROK kinases) that are the major mediators of pathogenesis in the mouse (PMID 23070557)
- We are extending these studies to identify strain-specific effectors that mediate resistance in humans. In human cellss, vacuoles containing susceptible strains (type II and III) of T. gondii are susceptible to ATG-mediated growth restriction while type I strains of T. gondii (type I) are largely resistant to this pathway (PMID 26350966). Additionally, type 5 and 10 strains from South America are resistant to interferon-mediated control in human cells
- We are using genetic crosses and QTL mapping to understand strain specific differences in how the ATG machinery recognizes the parasite-containing vacuole and to identify parasite derived factors that enable Toxoplasma to persist in IFN-γ activated human cells

New therapeutics for toxoplasmosis
- Current therapy for toxoplasmosis is complicated by allergic reactions, toxicity, and failure to eliminate the chronic infections that are characterized by slow growing bradyzoites found in tissue cysts. We are involved in screening for new therapeutic leads that are designed to target essential kinases like CDPK1 (PMID: 28933846) or to counteract known virulence factors such as ROP18 (PMID 27379343)
- In collaboration with the Broad Institute (https://www.broadinstitute.org/ ) and the HTS screening core at WUSM (http://htsc.wustl.edu/ ) we are involved in large scale phenotypic screening of small molecule libraries and developing screens to identify inhibitors that block replicating tachyzoites. We are also developing downstream screens to identify inhibitors that also affect and the slow-growing bradyzoites within tissue cysts. These studies involve high throughout screening of small molecular libraries, design and synthesis of new analogs to perform SAR studies, and genetic approaches to target identification
- For select leads, we are also working with medicinal chemists to improve bioavailability to treat acute and chronic toxoplasmosis in the murine model with the goal of identifying new leads for eventual clinical studies in human

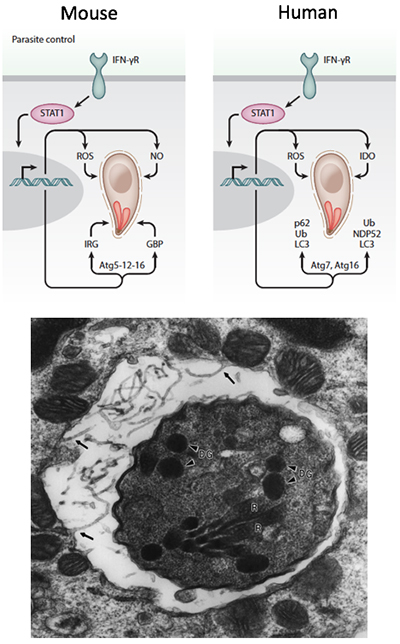
Cellular immunity and control of Toxoplasma
- Control of T. gondii in mouse and human relies on production of interferons (IFN), which signal in all nucleated cell types to induce various effectors that control intracellular pathogens. In both mouse and human cells, a subset of autophagy (ATG) proteins are required for IFNγ-mediated control of intracellular pathogens (PMID 26350966, 24931121, 18996346). The requirement of ATG proteins for IFNγ-mediated control spans both innate and adaptive immunity
- Although the requirement for IFNγ is well known, there are many downstream effectors that are unknown. We are using CRISPR/Cas9 for targeted gene deletion, proteomic analysis of protein complexes, and genome-wide screens to explore the following key questions:
- What effectors downstream of IFNγ are required for control of intracellular T. gondii in mouse and human cells?
- Why are core ATG proteins required for IFNγ effects? Are there novel ATG effectors that are required for control of T. gondii in human and mouse cells?
- What antigens are important for inducing protective responses and how are they processed and presented?
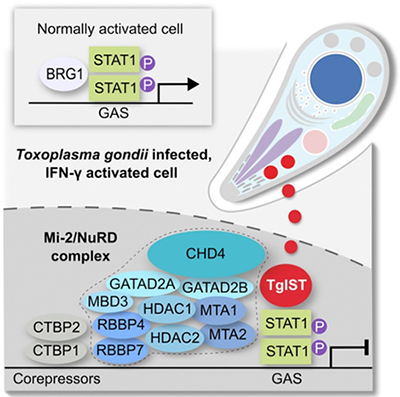
Discovery of novel Toxoplasma gondii secreted effectors
- The intracellular parasite T. gondii is an important human and veterinary pathogen that also serves as a model organism for less-easily studied apicomplexan parasites. As an obligate intracellular parasite, T. gondii secrets effectors that subvert diverse host cellular functions to promote growth and differentiation into a persistent stage in host tissues. These effectors hold the key for successful infection and subversion of the immune system
- Recently our lab has discovered a novel effector – TgIST that translocates across the parasitophorous vacuole into in the host cell nucleus, and blocks STAT1 signaling by interacting with the nucleosome-remodeling NuRD complex (PMID: 27414498). TgIST is one of a family of effector proteins that are released by the parasite, targeted to the host nucleus, where they alter chromatin and affect gene expression (PMID: 28404792)
- We are using a variety of biochemical, cellular, proteomic, and genetic approaches to further identify novel T. gondii secreted effectors and define their mode of action and significance in virulence and pathogenicity